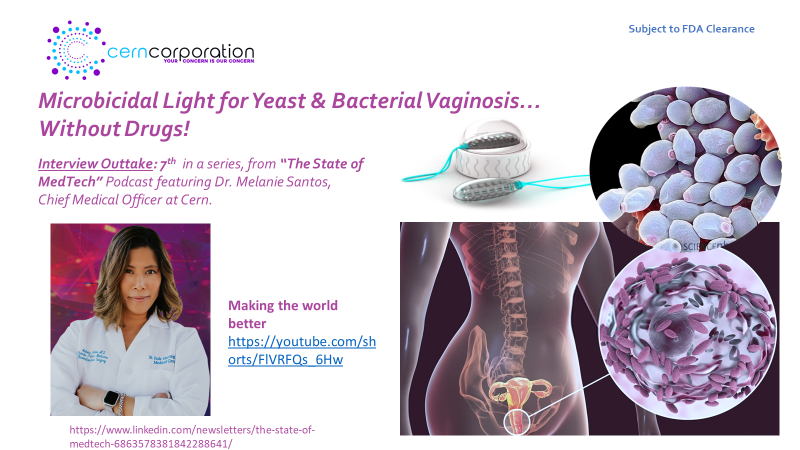
On behalf of “Team Cern”, thank you for all the tremendous support as we bring to market what so many believe to be a significant advancement in woman’s health, The Cern Device, for treatment of yeast and bacterial vaginosis…without drugs!
The journey to get to where we are today has been rewarding on so many levels. Hearing from so many who recognize significance of our work as significant, has been so motivational. The interest received has been off the chart both from those seeking access to the device as well as physicians who look to prescribe.
Investors and followers alike, we’d love to hear from you directly. Please shoot us an email at info@cerndevice.com and register in order to receive announcements.
Kind regards,
Gregg A. Klang
Cern Corporation, CEO