Cern Corporation, (The Cern Device™) is being recognized as one of nine cutting-edge companies that are transforming the healthcare industry.
(HEAL. LA) We’re excited to announce Heal.LA Demo Day (https://larta.org/ ), where we will showcase 9 cutting-edge companies, transforming the healthcare industry. Join us on May 9th from 11:00 am – 4:00 pm at BioscienceLA to meet the entrepreneurs and learn about their groundbreaking technologies.
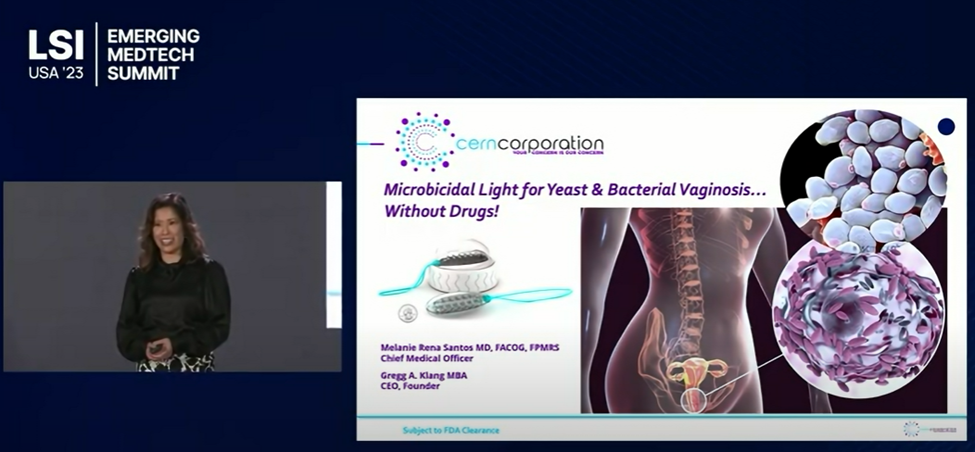
(Said Gregg Klang, Cern’s CEO) Cern is honored to be recognized by the LARTA Institute, and be included in this prestigious group of companies working to address significant issues in healthcare. Cern’s (The Cern Device™) patented use of low level microbicidal light to mitigate yeast and bacterial vaginosis is bringing interest both domestically and globally. According to the CDC, in the US alone, there are over 9M women under clinical care. This number is significantly under-reported. Why? The CDC only counts “recurrent” yeast infection when a woman is treated for a fourth occurrence within a year. That leaves those with 1-2-3 infections, not counted. With regard to bacterial vaginosis, of those afflicted, only 16% are symptomatic. The other 84% go without care….again, under-reported.
The Cern Device™…for those for whom drug based therapies are neither effective, appropriate, or desired!
Please help our quest to bring this new treatment to market as soon as possible. Invest now!