On behalf of “Team Cern”, thank you for all the support on our current round!
Please, if you haven’t already, finalize your investment in Cern to help bring this much needed therapeutic to market.
Validation!…Start Engine, accepts perhaps 50 companies from over 3000 that apply (About 1.5%). As they mentioned, the acceptance rate is, as they state is “Less then Harvard’s”. Cern’s current raise (Now above $420K) may put us in the top 10% of companies on the platform. Not bad!!!!!!!!!!!!
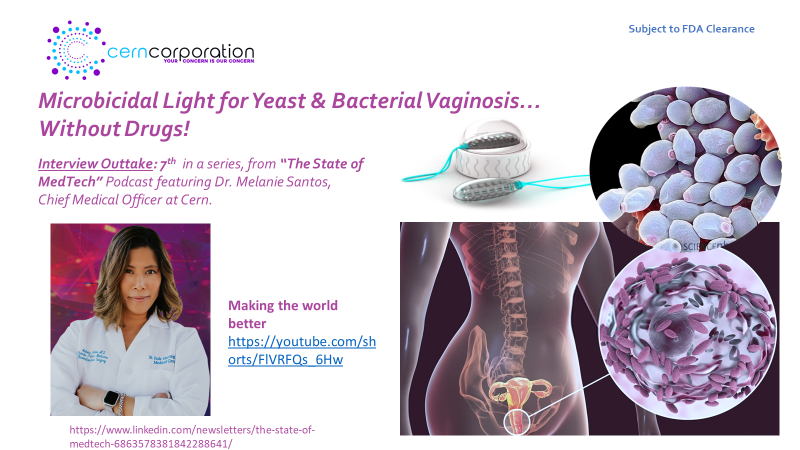
“The Why”? Because many (especially women and clinicians) understand, we have embarked on a significant, validated “Unmet Need” . One that is focused on the development of an effective non-drug treatment (yet compatible with) for yeast and bacterial vaginosis.
The Cern Device™ is designed for those women, for whom drug based standards of care are neither effective, appropriate, or…desired! Please continue to follow-us as we make tremndous progress.
Thank you again for all the support.
Kind regards, G