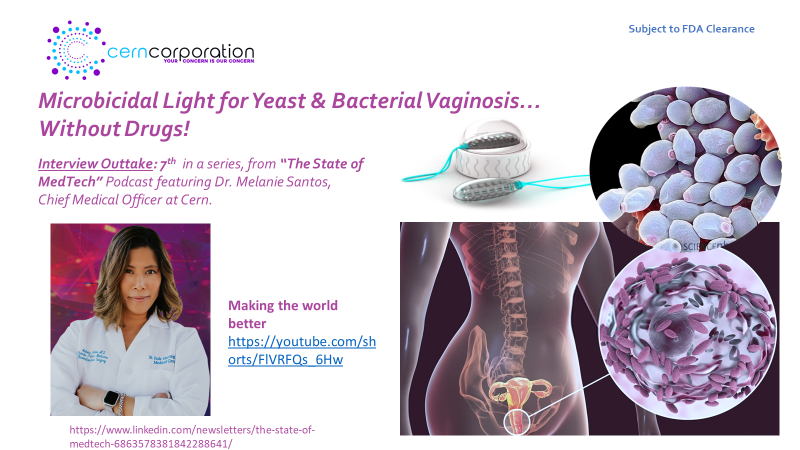
Interview Outtake 6: Decreasing the medical burden
More News
24 April 23

Cern recognised by LARTA Institute
Cern Corporation, (The Cern Device™) is being recognized as one of nine cutting-edge companies that are transforming the healthcare industry. (HEAL....
28 April 23

Cern funding ends tonight!
On behalf of “Team Cern”, thank you for all the support on our current round! Please, if you haven’t already, finalize...
25 November 25

HITLAB x Cern Partnership Announcement
New York, NY – November 5, 2025 — HITLAB, a global leader in digital health innovation and evidence generation, today announced...
