The newest episode of the Femtech Health Podcast is live!
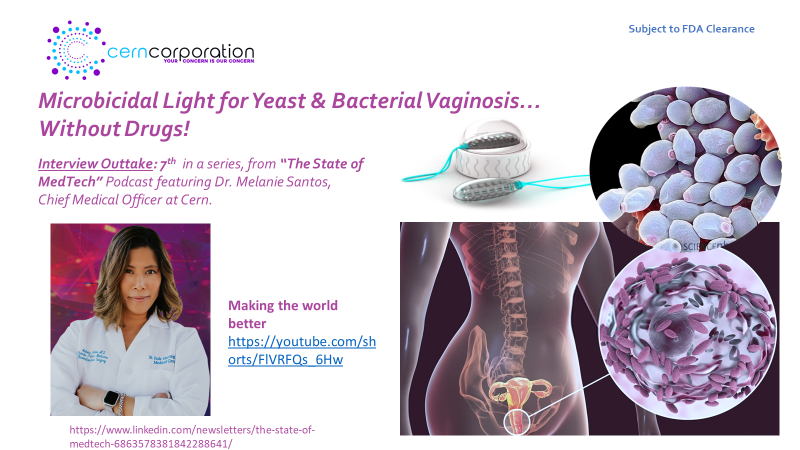
In this episode, we’re joined by Dr. Melanie Santos, a urogynecologist specializing in pelvic floor disorders and reconstruction. She is also the Medical Director of Pelvic Health at her healthcare system and serves as the Chief Medical Officer at Cern Corporation.
Dr. Santos discusses the prevalence of vaginitis, particularly bacterial vaginosis and yeast infections, which are among the most common reasons premenopausal women seek medical care. She explains the importance of proper diagnosis and treatment, as well as the risks associated with untreated infections. The conversation then shifts to the innovative Cern device, a light therapy treatment that could revolutionize the way these infections are managed without the need for antibiotics or antifungals.
You’ll learn:
– Bacterial Vaginosis Often Undiagnosed
– Untreated Infections Can Lead to Serious Complications
– Antibiotic-Free Light Therapy for Vaginal Infections
– Telehealth Expanding Women’s Healthcare Access
– Importance of Open Dialogue About Pelvic Health